In Vitro Diagnostics and IVD Quality Control Industry Data Book - In Vitro Diagnostics & IVD Quality Control Market Size, Share, Trends Analysis, And Segment Forecasts, 2022 - 2030
Grand View Research’s In Vitro Diagnostics and IVD quality control industry data book is a collection of market sizing & forecasts insights, regulatory & technology framework, pricing intelligence, volumetric analyses, competitive benchmarking analyses, macro-environmental analyses studies. Within the purview of the database, such information is systematically analyzed and provided in the form of summary presentations and detailed outlook reports on individual areas of research. The following data points will be included in the final product offering in two reports and one sector report overview.
Access the Global In Vitro Diagnostics and IVD Quality Control Industry Data Book, 2023 to 2030, compiled with details like trade data, pricing intelligence, and competitive benchmarking.
In Vitro Diagnostics Market Report Highlights
The global In Vitro Diagnostics Market size was estimated USD 111.67 Billion in 2021 and is expected to grow at a compound annual growth rate (CAGR) of 0.2% from 2022 to 2030.
- Molecular diagnostics is anticipated to grow at the fastest CAGR from 2023 to 2030 owing to the rising adoption and usage rate
- Reagents held the largest market share owing to the surge in demand for genetic testing and enhanced availability of technologically advanced diagnostic tests in lower and middle-income countries with unmet clinical needs
- The infectious diseases application segment held the largest market share owing to the large volume of testing for infectious diseases globally
- North America dominated the global market in 2022 owing to the high demand for novel technologies, a large pool of key players, high prevalence of diseases, and advanced healthcare infrastructure
Order your copy of Free Sample of “In Vitro Diagnostics and IVD Quality Control Industry Data Book - In Vitro Diagnostics & IVD Quality Control Market Size, Share, Trends Analysis, And Segment Forecasts, 2022 - 2030” Data Book, published by Grand View Research
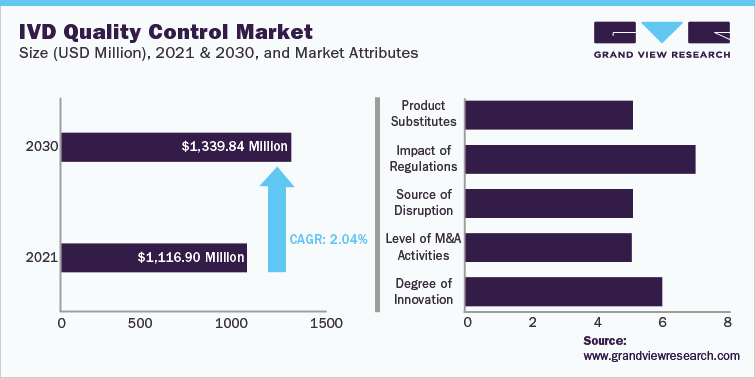
IVD Quality Control Market Report Highlights
The global IVD Quality Control Market size was estimated at USD 1.12 Billion in 2021 and is anticipated to grow at a compound annual growth rate (CAGR) of 2.0% from 2022 to 2030.
- In 2022, the clinical chemistry segment held the largest share of the market. The increasing demand for preventive medicine and the rapid transformation of clinical laboratories into highly automated and efficient businesses are some of the factors responsible for its high market share
- The quality control segment dominated the market in 2022 and is estimated to grow at the highest CAGR over the forecast period. Quality control in the research laboratory is concentrated on certifying the results provided to the individuals are precise
- The hospital segment held the largest share in 2022 due to the presence of highly advanced technology-based devices, such as Next Generation Sequencing (NGS), mass spectrophotometry, and microarrays, and the rising applications of the optimized quality-control procedures
- North America was a major revenue contributor in the global market in 2022 due to the presence of over 150,000 registered diagnostics laboratories. The clinical laboratories are required to provide accurate results and maintain the accuracy standards to retain their license to operate
- Many pharmaceutical companies are implementing the new draft guidance enforced by the U.S. FDA for data integrity on current Good Manufacturing Practices (cGMP)
- It provides information in relation to establishing robust operating procedures and strong management systems, obtaining high-quality raw materials, investigating deviations, and maintaining reliable diagnostic laboratories
Go through the table of content of In Vitro Diagnostics and IVD Quality Control Industry Data Book to get a better understanding of the Coverage and Scope of the study
Competitive Landscape
Market participants are updating their range of testing options for qPCR instruments by undertaking R&D initiatives for the development of kits that target emerging diseases or by entering into agreements with other kit manufacturing companies.
Key players operating in the In Vitro Diagnostics and IVD Quality Control industry are:
- Abbott
- bioMérieux
- Bio-Rad Laboratories
- Becton, Dickinson and Company
- Siemens Healthineers
- Qiagen
Check out more Industry Data Books, published by Grand View Research
About Grand View Research
Grand View Research, U.S.-based market research and consulting company, provides syndicated as well as customized research reports and consulting services. Registered in California and headquartered in San Francisco, the company comprises over 425 analysts and consultants, adding more than 1200 market research reports to its vast database each year. These reports offer in-depth analysis on 46 industries across 25 major countries worldwide. With the help of an interactive market intelligence platform, Grand View Research helps Fortune 500 companies and renowned academic institutes understand the global and regional business environment and gauge the opportunities that lie ahead.
Contact:
Sherry James
Corporate Sales Specialist, USA
Grand View Research, Inc.
Phone: 1-415-349-0058
Toll Free: 1-888-202-9519
Email: sales@grandviewresearch.com
Web: https://www.grandviewresearch.com/sector-reports-list
Follow Us: LinkedIn | Twitter

No comments:
Post a Comment