U.S. Intravenous Immunoglobulin Industry Overview
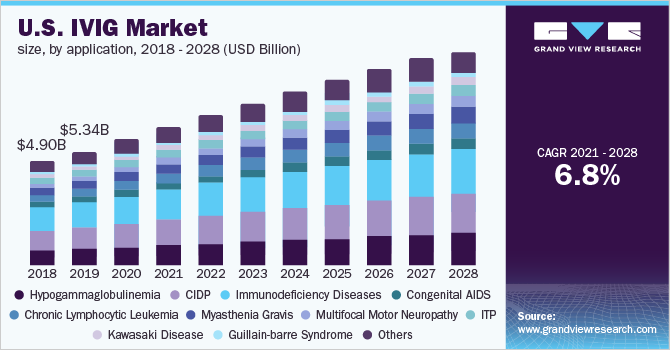
The U.S. intravenous immunoglobulin market size is expected to reach USD 10.24 billion by 2028, according to a new report by Grand View Research, Inc. It is expected to expand at a CAGR of 6.8% from 2021 to 2028. Robust pipeline, approval and launch of novel products, and increasing government initiatives are expected to be the major factors driving the market.
Robust pipeline and increasing approvals and launches of novel products are anticipated to support the market growth over the forecast period. For instance, in April 2019, U.S.-based ADMA Biologics, Inc. received U.S. FDA approval for its new drug Asceniv. It is an IVIG drug indicated for the treatment of primary humoral immunodeficiency disease. Similarly, in December 2019, CSL Behring announced receiving orphan drug exclusivity for Hizentra from the U.S. FDA to treat Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP). In another instance, in February 2021, Biotest AG announced that it was the first in Germany to manufacture an investigational hyper immunoglobulin from plasma protein for the COVID-19 treatment.
U.S. Intravenous Immunoglobulin Market Segmentation
Grand View Research has segmented the U.S. intravenous immunoglobulin market on the basis of application and distribution channel:
Based on the Application Insights, the market is segmented into Hypogammaglobulinemia, CIDP, Immunodeficiency Diseases, Congenital AIDS, Chronic Lymphocytic Leukemia, Myasthenia Gravis, Multifocal Motor Neuropathy, ITP, Kawasaki Disease, Guillain-barre Syndrome, and Others
- The immunodeficiency diseases segment dominated the market with a share of over 22.0% in 2020 owing to the existing patient base, coupled with the long-term therapy requirement associated with these diseases.
- The CIDP segment is anticipated to grow at a lucrative rate during the forecast period due to its user-friendly application and minimally invasive technique as compared to other treatment options.
- The multifocal motor neuropathy application segment is expected to register the fastest growth rate over the forecast period due to the high effectivity of IVIG in its treatment
Based on the Distribution Channel Insights, the market is segmented into Hospital Pharmacy, Specialty Pharmacy, and Others
- Hospital pharmacy held the largest share of over 58.0% in 2020 owing to a large number of hospitals and the easy availability of products. Hospital pharmacies include both in-patient and out-patient hospital pharmacies.
- The specialty pharmacy segment is expected to witness lucrative growth over the forecast period as specialty pharmacies facilitate easy treatment at home.
Key Companies Profile & Market Share Insights
Key players hold a major share in the market owing to their significant presence in the country. Emerging players are adopting organic growth strategies, such as novel drug development and product launches, to gain a significant market share.
Some prominent players in the U.S. Intravenous Immunoglobulin market include:
- Biotest AG
- Octapharma AG
- LFB Biotechnologies S.A.S
- China Biologics Products Holdings, Inc.
- Grifols, S.A.
- Kedrion Biopharma, Inc.
- CSL Behring
- McKesson Corporation
- Takeda Pharmaceutical Company Limited
- Bio Products Laboratory Ltd.
- Pfizer, Inc.
- ADMA Biologics, Inc.
Order a free sample PDF of the U.S. Intravenous Immunoglobulin Market Intelligence Study, published by Grand View Research.

No comments:
Post a Comment