Clinical Trial Supplies Industry Overview
The global clinical trial supplies market size is expected to reach USD 3.40 billion by 2030, registering a CAGR of 6.4% during the forecast period, according to a new report by Grand View Research, Inc. This growth can be attributed to the increasing volume of clinical trial studies coupled with the growing complexity of conducting these trials. The COVID-19 pandemic has created opportunities for local manufacturers. To enhance their foothold in the market, key companies are adopting various plans and policies to gain a higher market share. The clinical trial and pharmaceutical industry is anticipated to witness steady growth, which, in turn, will increase the demand for clinical trial supplies, thereby driving the market growth.
Clinical Trial Supplies Market Segmentation
Grand View Research has segmented the global clinical trial supplies market based on clinical phase, product/service, end-use, therapeutic use, and region:
Based on the Clinical Phase Insights, the market is segmented into Phase I, Phase II, Phase III and Others.
- The phase I segment is anticipated to witness the fastest CAGR of 6.9% during the forecast period. This is attributed to the large influx of phase I trials for COVID-19 treatment and prevention.
- Even though the phase I clinical trials segment is expected to grow at the fastest rate owing to growing R&D activities in the biotechnology space, the phase III segment is likely to dominate the market during the forecast period.
Based on the Product & Services Insights, the market is segmented into Manufacturing, Storage & Distribution and Supply Chain Management.
- The supply chain management segment held the largest market share of 46.7%.
- Globalization of clinical trials and an increase in the number of clinical sites are expected to drive the demand for supply chain management systems by life science companies & CROs.
- The adoption of mobile and supply chain management technology is expected to be the driving factor for segment growth.
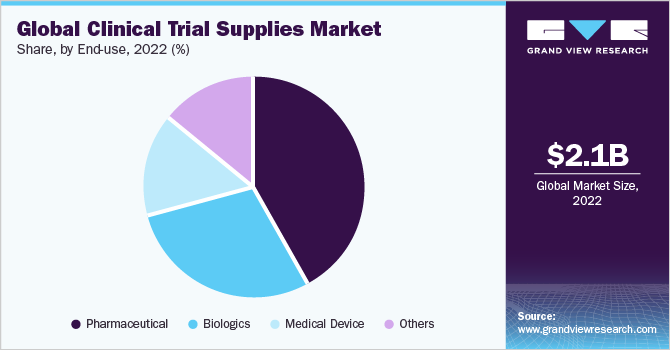
Based on the End-use Insights, the market is segmented into Pharmaceuticals, Biologics, Medical Device and Others.
- The pharmaceuticals segment held about 42.1% of the total market share in 2021 and is expected to exhibit a CAGR of 6.4% during the forecast period.
- Although pharmaceutical drugs account for the highest number of drugs in the clinical trial segment, the growing number of biological drugs is likely to have an impact on this number.
Based on the Therapeutic Use Insights, the market is segmented into Oncology, CNS, Cardiovascular, Infectious disease, Metabolic disorders and Others.
- In terms of therapeutic use, oncology is expected to be the fastest & dominant segment, exhibiting a CAGR of 7.2% over the forecast period. This is attributed to the presence of a huge R&D pipeline of oncology drugs.
- The majority of oncology drugs require temperature-sensitive distribution, which is expected to fuel the demand for cold chain distribution.
Clinical Trial Supplies Regional Outlook
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa (MEA)
Key Companies Profile & Market Share Insights
Geographical expansion and development of niche business units catering to the industry are being currently performed by various players.
Some prominent players in the global Clinical Trial Supplies market include:
- Almac Group Ltd.
- Biocair International Ltd.
- Catalent Pharma Solutions
- KLIFO A/S
- MOVIANTO (WALDEN GROUP)
- PCI Pharma Services
- Sharp Packaging Services
- Thermo Fischer Scientific, Inc.
- Marken
- PAREXEL International Corporation
- Patheon, Inc.
Other players present in the market
- Clinigen Group plc
- Merck Serono
- Chimerix
Order a free sample PDF of the Clinical Trial Supplies Market Intelligence Study, published by Grand View Research.

No comments:
Post a Comment