Bioanalytical Testing Services Industry Overview
The global bioanalytical testing services market size was estimated at USD 3.64 billion in 2021 and is anticipated to register a compound annual growth rate (CAGR) of 8.6% over the forecast period.
The bioanalytical testing method is used for the quantitative determination of drugs or metabolites in biological matrices, such as serum, blood, plasma, tissue, urine, and skin samples. The applications of such methods are bioavailability, pharmacology, bioequivalence, pharmacokinetic, and toxicology studies conducted in humans and animals. Also, with the outbreak of the COVID-19 pandemic, the market has witnessed high demand in the year 2020.
Gather more insights about the market drivers, restrains and growth of the Global Bioanalytical Testing Services market
Moreover, the increasing frequency of outsourcing R&D activities by the major pharmaceutical companies to focus on their core competencies is the vital impact rendering driver for this market. Furthermore, the economic efficiency offered by outsourcing rather than conducting an in-house study is expected to boost the demand over the forecast period. Innovation and new product development are expected to further boost the demand for these services. Competitive pressure and pricing concerns are driving companies to outsource such services. This, in turn, is expected to augment the overall market growth.
In addition, a growing focus on the development of advanced bioanalytical technologies for enhanced testing is expected to contribute to market growth. The development of biosimilars, combination products, and other innovative medicines has increased the demand for specific testing procedures. The coronavirus pandemic has resulted in disruptions in the supply chain of the overall pharmaceutical industry. However, the market for bioanalytical testing services responded well to the outbreak. The pandemic resulted in the expansion of R&D and manufacturing operations of key stakeholders leading to a surge in demand for these services.
Furthermore, several biopharmaceutical companies are using bioanalytical testing outsourcing services for drug development and validation of assays at both clinical and preclinical stages, thus supporting the growth of the market. The global transmission of coronavirus disease and the lack of effective treatments and vaccines are driving the need for early detection and widespread screening. This has also increased the usage of bioanalytical testing services for point-of-care diagnostic and minimizing the societal impact of the infection.
For instance, in January 2021, Eurofin’s bioanalytical services division announced the launch of a surrogate virus neutralization antibody assay against the COVID-19 virus. This assay is the first neutralizing antibody serology test authorized by the FDA as Emergency Use Authorization (EUA) for SARS-CoV-2-virus. Hence, such novel initiatives have led to the significant growth of the market during the year 2020. The market is further anticipated to witness lucrative growth over the forecast period owing to a significant surge in demand for such services post-pandemic.
Browse through Grand View Research's Pharmaceuticals Industry Research Reports.
Biosimilars Market - The global biosimilars market size was valued at USD 4.36 billion in 2016. It is anticipated to exhibit a CAGR of 34.2% during the forecast period.
Biologics Market - The global biologics market size was valued at USD 276.6 billion in 2015. These products represent cutting-edge research and also enable the latest scientific discoveries.
Market Share Insights
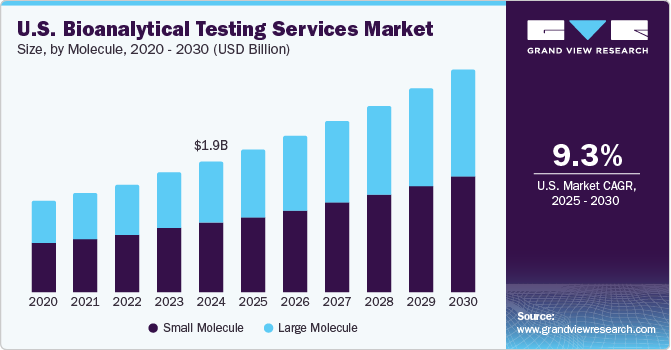
February 2021 - Nexelis, a provider of advanced assay development and laboratory testing services, has signed an asset purchase agreement with GlaxoSmithKline (GSK) to acquire GSK’s Good Clinical Laboratory Practices (GCLP)-certified clinical bioanalytical laboratory located in Marburg, Germany, with an aim to enhance its vaccines production capacities. In terms of molecules, the market is classified into small and large molecules.
January 2021 - Eurofin’s bioanalytical services division announced the launch of a surrogate virus neutralization antibody assay against the COVID-19 virus. This assay is the first neutralizing antibody serology test authorized by the FDA as Emergency Use Authorization (EUA) for SARS-CoV-2-virus.
Key Companies profiled:
Some prominent players in the global Bioanalytical Testing Services market include
- PPD, Inc.
- ICON Plc
- Charles River Laboratories International
- Covance, Inc.
- IQVIA
- Syneos Health
- SGS SA
- Toxikon
- Intertek Group Plc
- Pace Analytical Services LLC
Order a free sample PDF of the Bioanalytical Testing Services Market Intelligence Study, published by Grand View Research.

No comments:
Post a Comment