Preclinical CRO Industry Overview
The global preclinical CRO market size is expected to reach USD 9.67 billion by 2030, expanding at a CAGR of 7.9%, according to a new report by Grand View Research, Inc. The market is expected to show lucrative growth due to increasing R&D expenditure and rising outsourcing trends. The increasing volume of new drugs entering the preclinical phase is also anticipated to boost the market during the forecast period. However, the COVID-19 pandemic had temporarily affected the market owing to the shutdown of research sites due to the implementation of national lockdowns.
Preclinical CRO Market Segmentation
Grand View Research has segmented the global Preclinical CRO market on the basis of service, model type, end-use, and region:
Based on the Service Insights, the market is segmented into Bioanalysis and DMPK studies, Toxicology Testing, Compound Management, Chemistry, Safety Pharmacology and Others.
- The toxicology testing segment accounted for the largest revenue share of 25.48% of the global preclinical CRO market in 2021, owing to a rise in outsourcing of noncore preclinical CRO studies and high adoption of toxicology tests.
- The bioanalysis and DMPK studies segment is expected to register the fastest CAGR of 8.5% during the forecast period. The segment is expected to witness lucrative growth on account of a rise in the demand for pharmacokinetic services to support toxicology tests for IND-enabling studies.
Based on the Model Type Insights, the market is segmented into Patient Derived Organoid (PDO) Model and Patient derived xenograft model.
- The Patient Derived Organoid (PDO) Model segment held the largest share of 80.47% in 2021.
- The Patient derived xenograft model market has been analyzed to grow steadily during the forecast period. This is attributed to the growing number of CROs maintaining an in-house inventory of immunodeficient mice with patient-derived xenografts (PDXs).
- Furthermore, this type of analysis allows for researchers to co-relate the laboratory research with humans, owing to the maintenance of the original genetic makeup of the tumor cells.
- Also, the responses observed in clinical trials among patients have been found to correlate with the responses in these patient-derived xenografts, which in turn allows for a better safety profile and thus expedites the approval of New Drug Investigation (NDA).
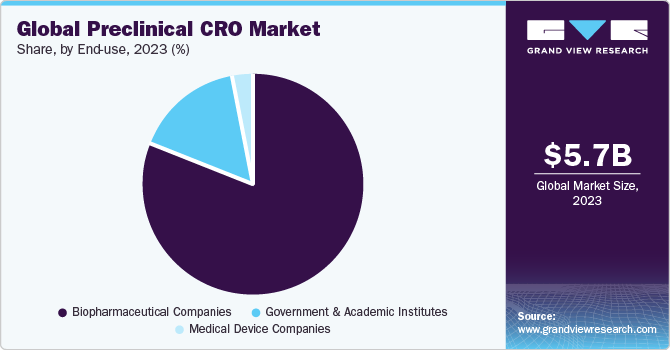
Based on the End-use Insights, the market is segmented into Biopharmaceutical Companies, Government and Academic Institutes and Medical Device Companies.
- The biopharmaceutical companies segment is expected to hold the largest market share of 80.89% in 2021.
- The government and academic institutes segment is estimated to register the fastest growth of 8.2% during the forecast period.
Preclinical CRO Regional Outlook
- North America
- Europe
- Asia Pacific
- Latin America
- MEA
Key Companies Profile & Market Share Insights
The market players in the Preclinical CRO industry are competitive and are constantly implementing strategies for improving their share in the market. Various strategies adopted by the players are acquisitions, new trial launches, and collaborations, among others.
Some prominent players in the Preclinical CRO market include
- Eurofins Scientific
- PRA Health Sciences, Inc.
- Wuxi AppTec
- Medpace, Inc.
- Charles River Laboratories International, Inc.
- Pharmaceutical Product Development (PPD), LLC
- SGS SA (SGS)
- Intertek Group Plc (IGP)
- Laboratory Corporation of America, Inc.
- Crown Bioscience
Order a free sample PDF of the Preclinical CRO Market Intelligence Study, published by Grand View Research.

No comments:
Post a Comment