COVID-19 Clinical Trials Industry Overview
The global COVID-19 clinical trials market size is expected to reach USD 9.9 billion by 2027, registering a CAGR of 9.5% during the forecast period, according to a new report by Grand View Research, Inc. An increasing number of deaths due to coronavirus is creating a need to develop effective treatments, thereby boosting the market growth. Also, the underlying economic profits for first movers are encouraging pharmaceutical players to invest in clinical trial studies for COVID-19.
The current pandemic poses an acute health risk to the entire population. A key to successfully fighting COVID-19 lies in clinical research. At present, almost all the major research-based pharmaceutical companies, many other biotechnology and pharmaceutical companies, as well as research institutes are engaged in a race to develop an effective treatment against coronavirus. There are currently 661 unique compounds in development against COVID-19, of which 292 are drugs, 173 are vaccines and 196 are antivirals. Factors such as globalization of clinical trials, demand for CROs to conduct clinical trials, and technological evolution, are further anticipated to drive growth.
COVID-19 Clinical Trials Market Segmentation
Grand View Research has segmented the global COVID-19 clinical trials market based on phase, product, and region:
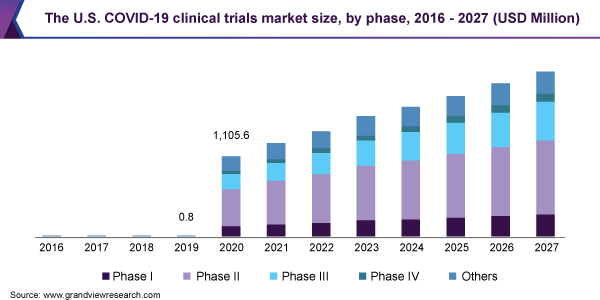
Based on the Phase Insights, the market is segmented into Phase I, Phase II, Phase III, Phase IV, and Others
- The Phase II segment accounted for the largest market share in 2020, it contributed to 30.3% of the global COVID-19 clinical trials market in 2020.
- This is attributed to the fact that the maximum number of therapeutics and vaccines in development are in Phase II.
Based on the Product Insights, the market is segmented into Therapeutics and Vaccines
- The vaccines segment held the largest market share of over 75% as of 2020. This is mainly due to the huge investments in the development of vaccines.
- The therapeutic segment is projected to witness the fastest CAGR of 10.0% over the forecast period. The growth is attributed to a large number of therapeutics are in development as compared to vaccines.
COVID-19 Clinical Trials Regional Outlook
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa (MEA)
Key Companies Profile & Market Share Insights
The key players are often involved in collaborations and partnerships to accelerate the production of vaccines and drugs against coronavirus.
Some prominent players in the global COVID-19 Clinical Trials market include:
- Moderna, Inc.
- GlaxoSmithKline plc
- Pfizer Inc.
- Johnson & Johnson
- Gilead Sciences Inc.
- INOVIO Pharmaceuticals
- AbbVie Inc.
- BioNTech SE
- Novavax
- Takeda
Order a free sample PDF of the COVID-19 Clinical Trials Market Intelligence Study, published by Grand View Research.

No comments:
Post a Comment