Medical Device Regulatory Affairs Industry Overview
The global medical device regulatory affairs market size was estimated at USD 4.5 billion in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 8.6% from 2021 to 2028. This growth can be attributed to rising demand for faster approval processes, a shifting regulatory landscape, and expansion in emerging fields such as therapeutics and diagnostics. Besides, the increasing complexity of medical devices and favorable government initiatives are also supporting the growth of the market for medical device regulatory affairs.
Right now, every aspect of the medical industry has been dominated by the COVID-19 pandemic. While the decline in economic activity has reduced revenue streams, demand for products such as ventilators and Personal Protective Equipment (PPE) has increased, mitigating the financial impact. As a result, regulatory agencies granted exemptions and emergency use authorizations quickly.
Gather more insights about the market drivers, restraints and growth of the Global Medical Device Regulatory Affairs market
The global regulatory landscape is changing rapidly, making it a complicated scenario for medical device firms. The conventional regulatory affairs are proving relatively unstable, with rules that might be changing for various products depending on what a manufacturer is selling. Exemptions related to COVID-19 have been issued by several countries around the globe. For instance, Australia issued an exemption for devices involved in diagnosis, confirmatory testing, prevention, monitoring, treatment, or alleviation of COVID-19.
Artificial intelligence and machine learning technologies have the potential to transform health by extracting a massive amount of data during healthcare delivery. Recently, numerous pre-market approvals have been granted through the FDA's pre-market pathway. One such example is the algorithm used in the Apple Watch's AF detector and diabetes management support tools that provide personalized treatment plans. The FDA has signified a different strategy that shifts the center of mass for certification, particularly for AI-related technologies, aside from a complete focus on the device or its performance and toward the company and its processes.
Besides, increasing cybersecurity threats and the financial impact of data breaches are making manufacturers of medical devices implement strategies to ensure that their products remain secure. This is being accompanied by government support. For instance, in October 2018, the FDA collaborated with the U.S. Department of Homeland Security to improve information sharing and collaboration to address cybersecurity risks, including less secure communication as well as prevention of unauthorized access when it comes to data transfer to and from the device.
Browse through Grand View Research's Healthcare Industry Related Reports
Regulatory Affairs Market - The global regulatory affairs market size was valued at USD 12.8 billion in 2021 and is anticipated to exhibit a compound annual growth rate (CAGR) of 8.7% over the forecast period.
Regulatory Affairs Outsourcing Market - The global regulatory affairs outsourcing market size was estimated at USD 6.5 billion in 2021 and is anticipated to expand at a compound annual growth rate (CAGR) of 8.9% from 2022 to 2030.
Medical Device Regulatory Affairs Industry Segmentation
Grand View Research has segmented the global medical device regulatory affairs market based on services, type, service provider, and region:
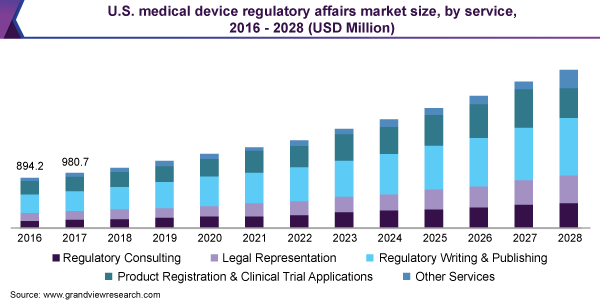
Medical Device Regulatory Affairs Service Outlook (Revenue, USD Million, 2016 - 2028)
- Regulatory Consulting
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Other Services
Medical Device Regulatory Affairs Type Outlook (Revenue, USD Million, 2016 - 2028)
- Diagnostics
- Therapeutics
Medical Device Regulatory Affairs Service Provider Outlook (Revenue, USD Million, 2016 - 2028)
- Outsource
- In-house
Medical Device Regulatory Affairs Regional Outlook (Revenue, USD Million, 2016 - 2028)
- North America
- Europe
- Asia Pacific
- Latin America
- MEA (Middle East & Africa)
Market Share Insights:
February 2020: Emergo has released 510(k) Builder, a new subscription-based software tool that can simplify and streamline the U.S. FDA’s submissions for the manufacturers of medical devices to get faster access to the market.
Key Companies profiled:
Some prominent players in the global Medical Device Regulatory Affairs Industry include
- ICON Plc
- Emergo
- Covance
- Freyr
- Promedica International
- Medpace
- IQVIA Holdings
- Intertek Plc
- SGS SA
- Integer Holdings
Order a free sample PDF of the Medical Device Regulatory Affairs Market Intelligence Study, published by Grand View Research.

No comments:
Post a Comment