Virtual Clinical Trials Industry Overview
The global virtual clinical trials market size was estimated at 7.8 billion in 2021 and is expected to expand at a compound annual growth rate (CAGR) of 5.7% from 2022 to 2030. The COVID-19 pandemic has significantly impacted the market. The market is majorly driven by a rise in R&D activities, the increasing healthcare digitization, as well as adoption of telehealth. Besides, technological advancements, alliances between clinical research organizations, pharmaceutical, and biotechnology companies as well as supportive government initiatives are anticipated to drive the market. The pandemic of COVID-19 is making the clinical trial industry change the way of conducting ongoing or upcoming trials.
As per the continuum clinical report published in April 2020, approximately 30% of the surveyed clinical trial places are projected to have a huge impact on recruiting patients for new trial studies as well as retaining already-enrolled patients compliant with their study schedules. Also, 81% of the European clinical trial study sites and 56% of the U.S. sites indicated that the patients are less likely to continue participating in studies. Besides, as of March 30, around 30 pharma or biotech companies have reported disruption to a trial as a result of the crisis. Virtual trials are also known as decentralized trials had a significant role to play in the COVID-19 crisis and are set to become a norm in the way trials and real-world studies are run.
Gather more insights about the market drivers, restraints, and growth of the Global Virtual Clinical Trials Market
A virtual method lets people take part in the trial from their homes ensuring research can continue even when site visits cannot, hence, representing a novel approach of collecting safety and efficacy data from participants of clinical studies. Virtual visits and remote patient monitoring of in-person site visits give participants a choice and peace of mind of not being exposed to unnecessary risks. The virtual studies enable sponsors to include a larger population in the study, thus improving recruitment, engagement, and retention. Also, it enables continuous real-time data collection through digital health technologies. Eventually, virtual connectivity, monitoring as well as management can significantly decrease the effort, time commitment, and burden on the participants, CRCs, and investigators.
Browse through Grand View Research's Medical Devices Industry Related Reports
HIV Clinical Trials Market - The global HIV clinical trials market size was valued at USD 1.1 billion in 2021 and is expected to expand at a compound annual growth rate (CAGR) of 6.3% from 2022 to 2030.
Cell And Gene Therapy Clinical Trials Market - The global cell and gene therapy clinical trials market size was valued at USD 7.3 billion in 2021 and is anticipated to exhibit a compound annual growth rate (CAGR) of 14.6% from 2022 to 2030.
Virtual Clinical Trials Industry Segmentation
Grand View Research has segmented the global virtual clinical trials market based on study design, indication, phase and region:
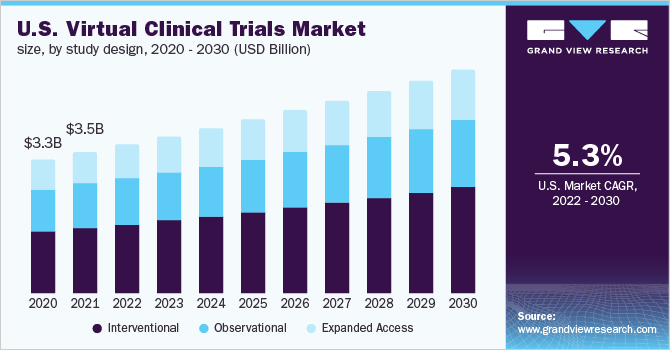
Virtual Clinical Trials Study Design Outlook (Revenue, USD Million, 2017 - 2030)
- Interventional
- Observational
- Expanded Access
Virtual Clinical Trials Indication Outlook (Revenue, USD Million, 2017 - 2030)
- CNS
- Autoimmune/Inflammation
- Cardiovascular Disease
- Metabolic/Endocrinology
- Infectious Disease
- Oncology
- Genitourinary
- Ophthalmology
- Others
Virtual Clinical Trials Phase Outlook (Revenue, USD Million, 2017 - 2030)
- Phase I
- Phase II
- Phase III
- Phase IV
Virtual Clinical Trials Regional Outlook (Revenue, USD Million, 2017 - 2030)
- North America
- Europe
- Asia Pacific
- Latin America
- MEA (Middle East & Africa)
Market Share Insights:
May 2020: Covance announced expanding its technology ecosystem to accelerate decentralized clinical trials adoption. The company is doing so through an alliance with Medable, a prominent software provider for digital clinical trials.
Key Companies profiled:
Some prominent players in the global Virtual Clinical Trials Industry include
- ICON, plc
- Parexel International Corporation
- IQVIA
- Covance
- PRA Health Sciences
- LEO Innovation Lab
- Medidata
- Oracle
- CRF Health
- Clinical Ink
- Medable, Inc.
- Signant Health
- Clinical Ink
- Halo Health Systems
- Croprime
Order a free sample PDF of the Virtual Clinical Trials Market Intelligence Study, published by Grand View Research.

No comments:
Post a Comment