Capnography Devices Industry Overview
The global capnography devices market size was valued at USD 565.3 million in 2021 and is expected to witness a compound annual growth rate (CAGR) of 9.9% from 2022 to 2030. Technological advancements, the prevalence of respiratory diseases, and supportive government initiatives are anticipated to boost the market growth over the forecast period. The growing adoption of capnography in the treatment of respiratory diseases, due to higher reliability and efficiency, is expected to improve the medical efficiency of these devices in patient monitoring. According to a 2020 report by the Office of Disease Prevention and Health Promotion, more than 25 million people in the U.S. are suffering from asthma. During the COVID-19 pandemic, market players, such as Medtronic, experienced a surge in the demand for capnography devices, among other respiratory system-related products.
This was mostly because capnography devices fall under the category of non-invasive remote monitoring devices and can be used to support patient monitoring during the treatment of COVID-19, as per the guidelines of the U.S. FDA. This positively impacted the market growth during the pandemic. Emergency medical service patients are frequently monitored with capnography any time pain medication, oxygen, or sedation is given along with if a patient is outfitted with an artificial airway. Elective surgical cases were annulled as COVID-19 flooded hospitals with infected patients. Thus, anesthesiologists and certified registered nurse anesthetists were transferred to intensive care units.
Gather more insights about the market drivers, restraints, and growth of the Global Capnography Devices market
These experts carried capnography knowledge into these units and implemented the technology to help support improved patient outcomes. The pandemic has enhanced the product sales as the demand for remote respiratory monitoring equipment increased, which, in turn, augmented the market growth. Capnography and pulse oximetry in combination is the monitoring standard of care in the operating room setting, and this combination is becoming more regularly used in several in-patient situations, comprising moderate sedation and patient-controlled analgesia. In addition, capnography has become the standard of care to confirm endotracheal intubation in all hospital settings.
The cost-effective and non-invasive features of capnography devices make it an ideal procedure for respiratory monitoring. The increasing geriatric populace and shift in lifestyle are positively impacting the market. Advancements in technology have paved the way for the innovation of portable, efficient, and automatic capnography devices. Moreover, strategic initiatives by key players have also fueled market growth. For instance, in April 2021, Masimo proclaimed that it has received FDA 510(k) clearance for its Radius PCG, a portable capnograph. The device comes with wireless Bluetooth connectivity. In September 2020, Capsule Technologies received FDA approval for its new connected capnography-monitoring technology. With this, the company enhanced its product portfolio.
Browse through Grand View Research's Medical Devices Industry Research Reports.
Ethylene Oxide Sterilization Services Market - The global ethylene oxide sterilization services market size was valued at USD 4.4 billion in 2022 and is projected to expand at a compound annual growth rate (CAGR) of 10.7% from 2023 to 2030.
Continuous Glucose Monitoring Devices Market - The global continuous glucose monitoring devices market was estimated to be valued at USD 7,816.8 million in 2022 and is estimated to expand at a compound annual growth rate (CAGR) of 4.4% from 2023 to 2030.
Capnography Devices Market Segmentation
Grand View Research has segmented the global capnography devices market on the basis of component, application, product, technology, end use, and region:
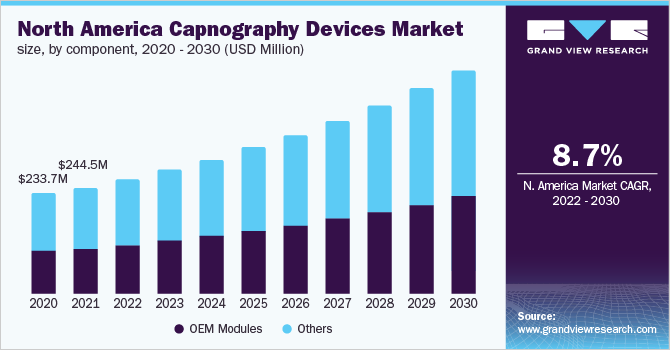
Capnography Devices Component Outlook (Revenue, USD Million, 2017 - 2030)
- OEM Modules
- Others
Capnography Devices Product Outlook (Revenue, USD Million, 2017 - 2030)
- Handheld
- Standalone
- Multiparameter
Capnography Devices Technology Outlook (Revenue, USD Million, 2017 - 2030)
- Mainstream
- Sidestream
- Microstream
Capnography Devices Application Outlook (Revenue, USD Million, 2017 - 2030)
- Emergency Medicine
- Pain Medicine
- Procedural Sedation
- Critical Care
- Others
Capnography Devices End-use Outlook (Revenue, USD Million, 2017 - 2030)
- Hospitals
- Ambulatory Care Centers
- Others
Capnography Devices Regional Outlook (Revenue, USD Million, 2017 - 2030)
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Market Share Insights
May 2020: Koninklijke Philips entered into a licensing agreement, through which, Philips integrated additional Masimo measurement technologies, such as NomoLine Capnography and O3 Regional Oximetry, into select IntelliVue MX-series multi-parameter monitors.
March 2020: Masimo acquired TNI Medical AG, a Germany-based company. Through this, it enhanced its presence in the European Union.
Key Companies profiled:
Some prominent players in the global Capnography Devices market include -
- Masimo
- Smiths Medical
- Dragerwerk AG & Co. KGaA
- Welch Allyn (Hill-Rom Holdings, Inc.)
- Koninklijke Philips N.V.
- Medtronic
- Nonin Medical, Inc.
- Nihon Kohden Corp.
- BD
- Diamedica Ltd.
Order a free sample PDF of the Capnography Devices Market Intelligence Study, published by Grand View Research.

No comments:
Post a Comment