Huntington's Disease Treatment Industry Overview
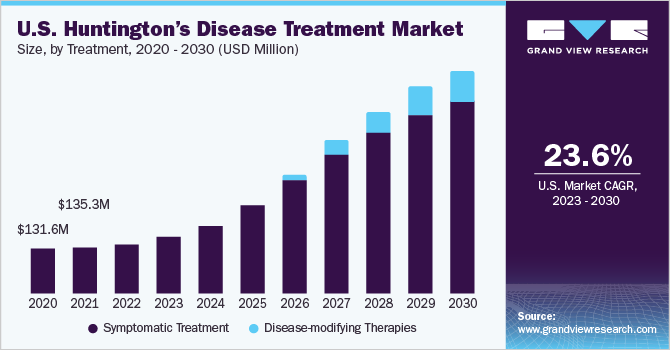
The global huntington’s disease treatment market size was valued at USD 362.0 million in 2021 and is expected to expand a compound annual growth rate (CAGR) of 19.6% from 2022 to 2030. The expected label expansion of Ingrezza for treating chorea associated with Huntington’s disease, high burden of HD in western countries, and strong product pipeline of disease-modifying therapies are anticipated to be major drivers for the market.HD roughly affects 30,000 people in North America, with a prevalence of 5.7 per 100,000 people. HD in children is considerably rarer, accounting for about 5% to 10% of all cases. It affects up to 10 out of 100,000 people in Europe.
Huntington's Disease (HD) is a progressive neurological condition caused by CAG expansions in the Huntingtin (Htt) gene. It affects one out of every 10,000 people in the United States. Healthy people have less than 35 CAG repetitions, whereas HD patients have CAG expansions ranging from 36 to 200. The prevalence varies by more than ten-fold between geographical locations, which can be related to variations in case ascertainment and diagnostic criteria. The Asian population has consistently had a lower prevalence, while Europe, North America, and Australia have a higher prevalence. Several small molecules in clinical development are focused on using immunomodulatory drugs to target the overactive immune system in HD.
Gather more insights about the market drivers, restraints, and growth of the Global Huntington's Disease Treatment market
Annexon, Inc. is engaged in the development of ANX005-an experimental monoclonal antibody that targets abnormal C1q activity in complement-mediated neurodegenerative diseases, such as HD. This innovative medication is administered intravenously (IV) and is meant to suppress C1q and the complete classical complement system. In November 2020, the company initiated a phase 2 clinical trial of ANX005 for treating HD patients. Stem cell therapy is also generating interest as a potential treatment for HD. Currently, Cellavita, in collaboration with AzidusPharma, is investigating stem cell therapy, CELLAVITA-HD in phase 2/ 3 ADORE-DH trial.
However, drug development for HD has faced significant obstacles as several therapies have failed to demonstrate efficacy or were associated with significant toxicity. In March 2021, F. Hoffmann-La Roche Ltd. discontinued the dosing in the phase III GENERATION HD1 study of tominersen in manifest HD. The conclusion was made on the basis of findings of an unblinded Independent Data Monitoring Committee's (iDMC) preplanned examination of data from the phase III research. During analysis of this data, no new or developing safety signals for tominersen were discovered.COVID-19 pandemic has had a slight negative impact on the market for Huntington’s disease treatment. However, the delays in conducting clinical trials due to the pandemic can delay the entry of pipeline candidates into the market.
Browse through Grand View Research's Pharmaceuticals Industry Research Reports.
Parkinson’s Disease Treatment Market - The global Parkinson’s disease treatment market size was valued at USD 4.28 billion in 2021 and is expected to expand at a compound annual growth rate (CAGR) of 12.1% from 2022 to 2030.
Inflammatory Bowel Disease Treatment Market - The global inflammatory bowel disease treatment market size was valued at USD 20.33 billion in 2022 and is expected to expand at a compound annual growth rate (CAGR) of 3.6% from 2023 to 2030.
Huntington’s Disease Treatment Market Segmentation
Grand View Research has segmented the global Huntington’s disease treatment market on the basis of treatment and region:
Huntington’s Disease Treatment Outlook (Revenue, USD Million, 2017 - 2030)
- Symptomatic Treatment
- Disease Modifying Therapies
Huntington’s Disease Treatment Regional Outlook (Revenue, USD Million, 2017 - 2030)
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Market Share Insights
September 2021: NeuExcell Therapeutics Inc entered into a partnership with Spark Therapeutics, Inc. (F. Hoffmann La Roche Ltd. owned subsidiary) for the development of gene therapy for treating HD patients. Under the terms of the agreement, NeuExcell Therapeutics is entitled to receive upfront license fees, R&D, and sales milestone payments of up to approximately USD 190 million and additional product royalties.
September 2021: Prilenia Therapeutics B.V. announced its participation in H.C. Wainwright 23rd Annual Global Investment Conference, Cantor Virtual Global Healthcare Conference, and European Huntington's Disease Network for showcasing the topline results of phase 3 candidatepridopidine. These healthcare conferences are an excellent platform for increasing the customer base.
Key Companies profiled:
Some of the prominent players in the Huntington’s disease treatment market include:
- Lundbeck A/S
- Teva Pharmaceutical Industries Ltd.
- Bausch Health Companies Inc.
- Hetero
- Lupin
- Hikma Pharmaceuticals PLC
- Reddy’s Laboratories Ltd.
- Sun Pharmaceutical Industries Ltd.
Order a free sample PDF of the Huntington's Disease Treatment Market Intelligence Study, published by Grand View Research.

No comments:
Post a Comment