Drug Device Combination Products Industry Overview
The global drug device combination products market size was valued at USD 118.13 billion in 2021 and is expected to expand at a compound annual growth rate (CAGR) of 8.8% from 2022 to 2030. The market is primarily driven by the growing patient and physician preference for minimally invasive procedures and consistent-dosing treatment alternatives. The pandemic positively impacted this market space as the use of certain combination products increased during the different waves of COVID-19. In November 2020, the U.S. Food and Drug Administration has issued an emergency use authorization (EUA) for baricitinib in combination with remdesivir in hospitalized adults and pediatric patients two years of age and older who require supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation for suspected or laboratory-confirmed COVID-19 (ECMO).
Article 117 of the Medical Devices Regulation (MDR) requires manufacturers to obtain a Notified Body Opinion before placing drug-device combination goods on the market as an integral device and marketing them as a "medicinal product" (NBOp). The notified body then certifies that the device complies with the relevant General Safety and Performance Requirements (GSPR) and sends the manufacturer an NBOp Report to include in the Market Authorization Application (MAA). Autoinjectors, inhalers, pre-filled nebulizers, pre-filled pens, prefilled syringes, and transdermal patches are examples of drug-device combination items that require NBOp.
Gather more insights about the market drivers, restraints, and growth of the Global Drug Device Combination Products market
The subsequent increase in the adoption of these products can be credited to associated benefits such as reduced pain levels, better patient outcomes, reduced hospital stay, and overall healthcare cost-efficiency. Other advantages include synergistic effects facilitating multi-target treatment, improved tolerance levels, simplification of dosage regime, and improved symptomatic and pharmacokinetic profiles. These benefits are expected to propel the demand for these systems and present the market with numerous growth opportunities.
In addition, frequent intervention by government health organizations to ensure high patient safety is predominantly driving the market. To address the challenges associated with drug-device combination products as well as facilitate proper and consistent regulation, the U.S. FDA has established the Office of Combination Products (OCP). Moreover, unprecedented growth in the development of clinical drugs and devices by large pharmaceutical companies is believed to meet the demand for advanced medication delivery technologies. This has resulted in extensive product pipelines and maximized commercial returns on already established products, thereby serving as a significant driving factor for the industry.
Browse through Grand View Research's Medical Devices Industry Research Reports.
Intravenous Infusion Pump Market - The global intravenous infusion pump market size was estimated at USD 5.3 billion in 2022 and is expected to expand at a compound annual growth rate (CAGR) of 8.3% from 2023 to 2030.
Inhaled And Intranasal Products Contract Service Providers Market - The global inhaled and intranasal products contract service providers market size was valued at USD 2.4 billion in 2022 and is expected to expand at a compound annual growth rate (CAGR) of 8.5% from 2023 to 2030.
Drug Device Combination Products Market Segmentation
Grand View Research has segmented the global drug device combination products market based on product and region:
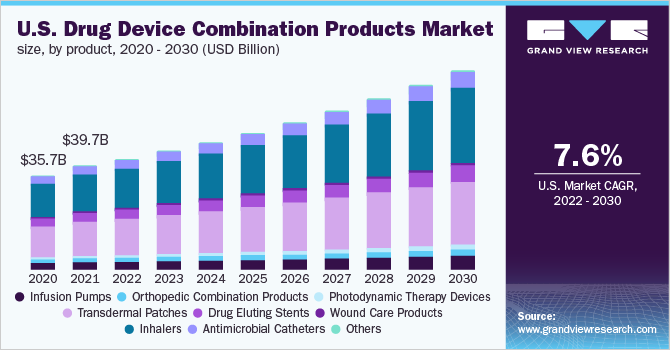
Drug Device Combination Product Outlook (Revenue, USD Billion, 2018 - 2030)
- Infusion Pumps
- Orthopedic Combination Products
- Photodynamic Therapy Devices
- Transdermal Patches
- Drug Eluting Stents
- Wound Care Products
- Inhalers
- Antimicrobial Catheters
- Others
Drug Device Combination Products Regional Outlook (Revenue, USD Billion, 2018 - 2030)
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)
Market Share Insights
February 2016: Medtronic introduced the New Resolute Onyx drug-eluting stent, commercially available in different sizes in Europe, thereby enhancing the company’s current product offerings
Key Companies profiled:
Some of the prominent players in the global Drug Device Combination Products market include:
- Abbott
- Terumo Corporation
- Stryker Corporation
- Mylan N.V.
- Medtronic
- Allergan plc
- Boston Scientific Corporation
- Novartis AG
- C.R. Bard
- Teleflex Incorporated
- W. L. Gore & Associates, Inc.
Order a free sample PDF of the Drug Device Combination Products Market Intelligence Study, published by Grand View Research.

No comments:
Post a Comment