Clinical Trial Imaging Industry Overview
The global clinical trial imaging market size was estimated at USD 851.8 million in 2021and is expected to expand at a compound annual growth rate (CAGR) of 8.7% from 2022 to 2030. Mounting biotechnology and pharmaceutical industries combined with increased research and development investments for developing new drugs to treat diseases is expected to drive the market industry growth. Medical imaging plays an active role in the clinical development of novel life science products. Although the medical imaging industry is in a constant state of fluctuation, the biotechnology, and pharmaceutical industries continue to increase. This can be attributed to the enhanced investment in medical imaging companies and mergers and acquisitions, supported by the adoption of innovative imaging technologies to support clinical trials for medical devices.
However, the high cost of machinery and their installations, and the enormous cost of clinical trials may limit the market growth during the forecast period. Advancements in technology are bringing substantial improvements to the collection, evaluation, and submit clinical trial imaging data. Technology-enabled imaging, especially image analysis software, provides various benefits to clinical studies such as consistency, data accuracy, adaptability as well as compliance. For instance, image analysis software is used to direct and manage a reader via analysis of imaging time points.
Gather more insights about the market drivers, restraints, and growth of the Global Clinical Trial Imaging market
The outbreak of COVID-19 has adversely impacted the healthcare system in most countries, leading to a disruption in medical studies, research activities, and reduced sponsorship for research involving clinical trials. The pandemic has hampered the clinical trial timeline as numerous ongoing studies were delayed and planned studies were canceled. Unfavorable changes in regulations and guidelines, supply chain disruption, recruitment challenges for clinical trials, fear of viral spread, and shutting down of most manufacturers during lockdown have also adversely impacted the market. However, the introduction of virtual imaging trials during the COVID-19 pandemic is expected to open new avenues for the adoption of these devices. The development of advanced computational models helps has helped in better assessment of CT and radiography images which are expected to help in the early diagnosis of COVID-19 in patients. The clinical trial imaging market is said to bounce back by 2022 Q2, supported by the rise in R&D activities and improvement in supply and distribution channels.
Increased use of imaging technology along with the enhanced power of computing is expected to drive the usage of imaging in clinical trials. The Quantitative Imaging Biomarkers Alliance (QIBA) protocol has come up with standardized methods and imaging procedures with uniform procedures to be implemented for attaining statistical and precise endpoints in clinical trials.
For improving image assessment and capturing, numerous technology patents have been filed. Also, patented technologies are being provided by the imaging core lab players that are expected to aid the pharmaceutical companies in minimizing development timelines. For instance, a reading tool named Assessa, by IXICO aids in improved decision-making in clinical trials for diseases associated with memories such as schizophrenia, Parkinson’s, and Alzheimer’s disease, and neurological disorders like dementia and cognitive impairment.
Browse through Grand View Research's Medical Devices Industry Research Reports.
Medical X-ray Generators Market - The global medical X-ray generators market size was valued at USD 2.01 billion in 2022 and is expected to expand at a compound annual growth rate (CAGR) of 5.1% from 2023 to 2030.
Digital X-ray Devices Market - The global digital X-ray devices market size was valued at USD 3.6 billion in 2022 and is expected to expand at a compound annual growth rate (CAGR) of 3.0% from 2023 to 2030.
Clinical Trial Imaging Market Segmentation
Grand View Research has segmented the global clinical trial imaging market based on service, application, end-use, and region:
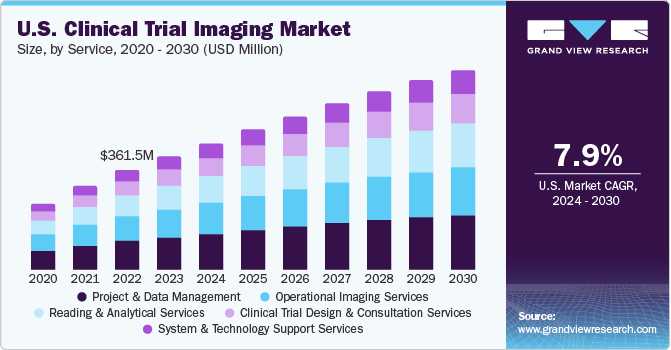
Clinical Trial Imaging Service Outlook (Revenue, USD Million, 2018 - 2030)
- Clinical Trial Design and Consultation Services
- Reading and Analytical Services
- Operational Imaging Services
- System and Technology Support Services
- Project and Data Management
Clinical Trial Imaging Application Outlook (Revenue, USD Million, 2018 - 2030)
- NASH
- CKD
- Diabetes
- Cardiovascular Diseases
- Musculoskeletal
- Oncology
- Gastroenterology
- Pediatrics
- Others
Clinical Trial Imaging End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Biotechnology and Pharmaceutical companies
- Medical Devices Manufacturers
- Academic and Government Research Institutes
- Contract Research Organizations (CROs)
- Others
Clinical Trial Imaging Regional Outlook (Revenue, USD Million, 2018 - 2030)
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Market Share Insights
February 2020: ICON plc acquired MedPass International, a European medical devices CRO, reimbursement, and regulatory consultancy. This acquisition has reportedly helped in the expansion of the medical device and diagnostic research services of ICON in Europe.
June 2018: Bioclinica introduced the SMART technology suite available with Medical Imaging, Interactive Response Technology (IRT), and Electronic Data Capture (EDC) to submit, manage, investigate, report & transfer medical image data compliant with universal data privacy and global requirements.
Key Companies profiled:
Some of the prominent players in the global clinical trial imaging market include:
- IXICO plc
- Navitas Life Sciences
- Resonance Health
- ProScan Imaging
- Radiant Sage LLC
- Medpace
- Biomedical Systems Corp
- Cardiovascular Imaging Technologies
- Intrinsic Imaging
- BioTelemetry
Order a free sample PDF of the Clinical Trial Imaging Market Intelligence Study, published by Grand View Research.

No comments:
Post a Comment