Continuous Manufacturing Market In Pharmaceuticals & Biopharmaceuticals Overview
The global continuous manufacturing market in pharmaceuticals and biopharmaceuticals size was valued at USD 919.7 million in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 13.85% from 2021 to 2027. In recent years, continuous manufacturing has gained traction as a viable solution within the pharmaceutical industry. This is primarily to minimize the pressure pertaining to reduce drug development time and costs while maintaining the supply and quality of the final product. As a result, the continuous manufacturing market is anticipated to witness healthy growth in the coming years. Continuous manufacturing enables the therapeutics developers to keep pace with the changing market demands. It allows a seamless drug production process from raw materials to the final product. Moreover, the risk of human error is reduced because it entails a smaller number of human resources in the entire production process. Thus, it can be effectively adopted for the production of precision medicines and drugs with breakthrough therapy designations.
The pharma and biopharma industry is engaged in strategic alliances with academic institutes to develop commercial continuous processing technologies as a strategy toward a completely continuous system. Several publications have reported the development of a fully integrated end-to-end commercial continuous process for biologics production. This is anticipated to boost the implementation of this technology in commercial product development.
Gather more insights about the market drivers, restraints and growth of the Global Continuous Manufacturing Market in Pharmaceuticals & Biopharmaceuticals
Novartis and other pharma/biopharma companies are engaged in transforming the drug product process by supplanting conventional batch-based systems with continuous manufacturing. For instance, in October 2019, Sanofi opened its first digitally-enabled, continuous manufacturing facility in Massachusetts. The facility was established to accelerate biologics production. In addition, regulatory authorities are making focused efforts to encourage continuous processing.
Both the U.S. FDA and the European Medicines Agency (EMA) are making amendments in guidelines or releasing new statements with the increasing investment in this market. The FDA has released a statement titled “modern approach to advanced pharmaceutical manufacturing” in February 2019 that elaborates the ongoing innovations in the market. Active participation of regulatory bodies is anticipated to significantly spur the market revenue in the coming years.
Browse through Grand View Research's Healthcare Industry Related Reports
Active Pharmaceutical Ingredients Market - The global active pharmaceutical ingredients market size was valued at USD 209.7 billion in 2021 and is expected to expand at a compound annual growth rate (CAGR) of 6.0% from 2022 to 2030.
High Potency Active Pharmaceutical Ingredients Market - The global high potency active pharmaceutical ingredients market size was valued at USD 20.39 billion in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 8.4% from 2021 to 2028.
Continuous Manufacturing Market In Pharmaceuticals & Biopharmaceuticals Segmentation
Grand View Research has segmented the global continuous manufacturing market in pharmaceuticals and biopharmaceuticals on the basis of therapeutics type, application, formulation, mode, scale, product, and region:
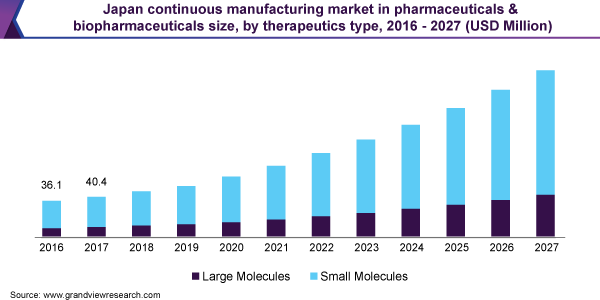
Continuous Manufacturing Market In Pharmaceuticals & Biopharmaceuticals Therapeutics Type Outlook (Revenue, USD Million, 2016 - 2027)
- Large Molecules
- Small Molecules
Continuous Manufacturing Market In Pharmaceuticals & Biopharmaceuticals Application Outlook (Revenue, USD Million, 2016 - 2027)
- Finished Product Manufacturing
- API Manufacturing
Continuous Manufacturing Market In Pharmaceuticals & Biopharmaceuticals Formulation Outlook (Revenue, USD Million, 2016 - 2027)
- Solid Formulation
- Liquid & Semi-solid Formulation
Continuous Manufacturing Market In Pharmaceuticals & Biopharmaceuticals Mode Outlook (Revenue, USD Million, 2016 - 2027)
- In-house
- Contract
Continuous Manufacturing Market In Pharmaceuticals & Biopharmaceuticals Scale Outlook (Revenue, USD Million, 2016 - 2027)
- Preclinical
- Clinical
- Commercial
Continuous Manufacturing Market In Pharmaceuticals & Biopharmaceuticals Product Outlook (Revenue, USD Million, 2016 - 2027)
- Integrated Systems
- Semi-continuous Systems
- Others
Continuous Manufacturing Market In Pharmaceuticals & Biopharmaceuticals Regional Outlook (Revenue, USD Million, 2016 - 2027)
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Market Share Insights
September 2020: Continuus Pharmaceuticals signed a contract with Roche to establish an end-to-end integrated CM process for the development of the latter’s new antiviral drug. It will include API manufacturing and drug product formulation.
April 2020: Danaher completed the acquisition of the bioprocessing unit of GE Healthcare. With this acquisition, it became a complete end-end supplier of bioprocessing solutions and was able to boost competitive rivalry with other key bioprocessing leaders, such as Sartorius.
Key Companies profiled:
Some prominent players in the global Continuous Manufacturing Market In Pharmaceuticals & Biopharmaceuticals include
- Thermo Fisher Scientific, Inc.
- Pall Corporation
- Applikon Biotechnology
- Sartorius Stedim Biotech
- GEA Group Aktiengesellschaft
- Corning Incorporated
- Merck KGaA
- Glatt GmbH
- Repligen Corporation
- Eppendorf AG
- Electrolab Biotech Ltd.
- Solesis Medical
- Scott Equipment Company
- B. Bohle
- LONZA
- 3M
- CellGenix GmbH
- Boehringer Ingelheim International GmbH
- Bio-Rad Laboratories
- Avantor, Inc.
- Ajinomoto Bio-Pharma
- GlaxoSmithKline
- Continuus Pharmaceuticals
- Arcinova
- Amgen
Order a free sample PDF of the Continuous Manufacturing Market In Pharmaceuticals & Biopharmaceuticals Intelligence Study, published by Grand View Research.

No comments:
Post a Comment