Clinical Trials Support Services Industry Overview
The global clinical trials support services market was estimated at USD 19.4 billion in 2021 and is projected to expand at a compound annual growth rate (CAGR) of 7.5% from 2022 to 2030. The global market is projected to expand rapidly due to the rising demand for trials in emerging economies, rising R&D investment, and an increasing number of contract research organizations (CROs). The pharmaceutical firms’ (R&D) venture has been steadily increasing every year, largely due to patent expirations. An ordinary patent terminates after 20 years; in the pharmaceutical area, there is an arrangement that provides for the entry of a generic version of the medication into the market following a time of 10 years. Thus, firms are boosting their R&D interests to speed the advancement of drugs, subsequently extending the whole market.
Clinical trials support services are quite useful in the event of a drug, assay design, and clinical testing. It also covers tasks such as strengthening clinical test locations, securing and storing research medicines, drug dosage calculation, and kit handling. Preclinical groundwork and research are provided by clinical test support services, which include clinical test site assistance, obtaining and storing study drugs, blinding of study drugs, patient recruiting, coordination, and reconciliation of returned medications.
Gather more insights about the market drivers, restraints, and growth of the Global Clinical Trials Support Services Market
The clinical trial sector underwent significant changes in 2021. With the introduction of COVID-19 vaccines, numerous clinical trials that had been halted due to the pandemic were re-initiated. In the first quarter of 2021, around 44% more clinical trials commenced compared to the first quarter of 2020. Although in-person clinical trials were once again viable, adoption of new technologies remained high, with 74% of sponsors adopting remote monitoring and 77% of sites using eRegulatory software.
Many software tools for data management are referred to as “Clinical Data Management” (CDM) systems. Multicentric trials require CDM systems to handle massive volumes of data. The majority of CDM systems utilized by pharmaceutical companies are commercial, but there are a few free-source tools accessible as well. Oracle Clinical, Clintrial, Oracle Clinical, Macro, RAVE, and eClinical Suite are common CDM tools. Maintaining an audit record of data management actions is important in regulatory submission studies. These CDM tools help ensure the audit trail and manage discrepancies. The CDM activities include data collection, CRF tracking, CRF annotation, database design, data entry, medical coding, data validation, discrepancy management, and database lock.
Browse through Grand View Research's Medical Devices Industry Related Reports
Healthcare Contract Research Organization Market - The global healthcare contract research organization market size was valued at USD 42.3 billion in 2021 and is anticipated to expand at a compound annual growth rate (CAGR) of 6.5% from 2022 to 2030.
Clinical Trials Market - The global clinical trials market size was valued at USD 47.0 billion in 2021 and is expected to expand at a compound annual growth rate (CAGR) of 5.8% from 2022 to 2030.
Clinical Trials Support Services Industry Segmentation
Grand View Research has segmented the clinical trials support services market based on service type, phase, sponsor, and region:
Clinical Trials Support Service Outlook (Revenue, USD Million, 2018 - 2030)
- Clinical trial site management
- Patient recruitment management
- Data management
- Administrative staff
- IRB
- Others
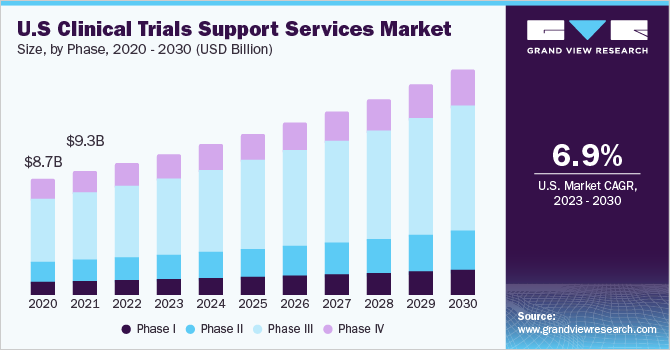
Clinical Trials Support Services Phase Outlook (Revenue, USD Million, 2018 - 2030)
- Phase I
- Phase II
- Phase III
- Phase IV
Clinical Trials Support Services Sponsor Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceutical & Biopharmaceutical companies
- Medical Devices
- Others
Clinical Trials Support Services Regional Outlook (Revenue, USD Million, 2018 - 2030)
- North America
- Europe
- Asia Pacific
- Latin America
- MEA (Middle East & Africa)
Market Share Insights:
April 2019: The WuXi AppTec acquired a clinical research services company, Pharmapace, Inc., to expand its Biometrics offerings in clinical research with data management, statistical programming, clinical data integration, biostatistics, and medical writing.
Key Companies profiled:
Some prominent players in the global Clinical Trials Support Services Industry include
- Charles River Laboratories Inc.
- Eurofins Scientific
- IQVIA
- Syneos Health Inc.,
- The Pharmaceutical Product Development LLC
- Icon PLC
- WuXi AppTec
- LabCorp
- Alcura
- Parexel International
Order a free sample PDF of the Clinical Trials Support Services Market Intelligence Study, published by Grand View Research.

No comments:
Post a Comment