Point Of Care Diagnostics Industry Overview
The global point of care diagnostics market size was valued at USD 37.03 billion in 2021 and is expected to expand at a compound annual growth rate (CAGR) of 6.8% from 2022 to 2030. The market is set to gain momentum in the coming years on account of the increase in funding from multiple sources, the rising prevalence of target diseases, and the need to address the recent outbreak of coronavirus. The ongoing COVID-19 pandemic had an enormous impact on the global industry in 2021 and the COVID-19 effect is anticipated to reflect in the projected tenure. For instance, in September 2021, the South Korean Department of Defense awarded USD 626 million to Celltrion as a part of the procurement contract for DiaTrust COVID-19 Ag Rapid Test.
However, the pandemic disrupted the supply chain and restricted the manufacturers to receive substantial raw material from across borders. An increase in funding by multiple sources, including the U.S. Department of Defense (DOD), the NIH, and private foundations like the Bill & Melinda Gates Foundation, is expected to drive the POCT market. For instance, Grand Challenges Canada and the Bill & Melinda Gates Foundation have launched a joint initiative for POC diagnostics. These organizations are involved in the development and integration of different diagnostic components into interoperable POC platforms with a “plug-and-play” characteristic feature and the capability of running diverse tests from various developers. Research is carried out to incorporate each test on a common platform with a single interface, enabling analysis of different sample specimens, with various approaches for analysis.
Gather more insights about the market drivers, restraints, and growth of the Global Point Of Care Diagnostics market
The integration of digital technologies is expected to exhibit a powerful impact on the expansion of POC solutions in limited resource settings. Increasing usage of telehealth as new normal is a critical go-to-market strategy for POCT players. As per the U.S. Centers for Disease Control and Prevention, approximately 95% of health centers in the U.S. provided telehealth services during the pandemic. Therefore, the expansion of remote patient monitoring systems such as PixCell Medical, a POC technology for CBC tests, is expected to positively impact the market growth.
The increasing importance of POC diagnostics in environmental monitoring and public health also demands the integration of technologies that facilitate easy networking, further making it convenient for healthcare professionals to interpret test results accurately. Companies are developing affordable POC with high specificity and sensitivity. For instance, in March 2022, Everything Genetic received approval from the U.K. regulatory bodies for selling COVID-19 Antigen Tests in the U.K. market. The company sells lateral flow devices at GBP 2 for single kits, this is expected to surge the POC diagnostics market growth, especially in developing economies.
Browse through Grand View Research's Clinical Diagnostics Industry Research Reports.
Body Fluid Collection And Diagnostics Market - The global body fluid collection and diagnostics market size was valued at USD 30.19 billion in 2022 and is projected to expand at a compound annual growth rate (CAGR) of 7.5% from 2023 to 2030.
Liver Cancer Diagnostics Market - The global liver cancer diagnostics market size was valued at USD 8,718.8 million in 2022 and is anticipated to expand at a compound annual growth rate (CAGR) of 6.67% by 2030.
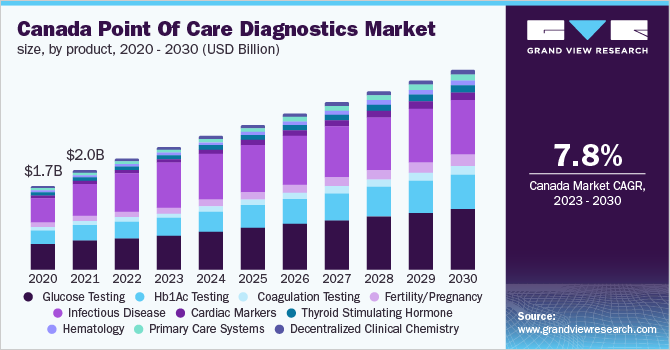
Point Of Care Diagnostics Market Segmentation
Grand View Research has segmented the global point of care diagnostics market based on product, end-use, and region:
Product Outlook (Revenue, USD Million, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Urinalysis/Nephrology
End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
Regional Outlook (Revenue, USD Million, 2018 - 2030)
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Market Share Insights
March 2022: Una Health announced to partner with Siemens Healthineers for expanding the distribution channel of Atellica VTLi Patient-side Immunoassay Analyser in the U.K. This product launch demo exhibited the company's commitment to POC diagnostics and critical care.
March 2022: Visby Medical announced to receive funding of USD 25.5 million from the U.S. Biomedical Advanced Research and Development Authority in order to develop a rapid flu-COVID-19 PCR test for home use. At present, the test is in the under-developing phase and the design is ready as a PCR device that can detect COVID-19, influenza A, and B from a single sample.
Key Companies profiled:
Some prominent companies in the global point of care diagnostics market include:
- F. Hoffmann-La Roche Ltd.
- Danaher
- BD
- Qiagen
- Abbott
- Siemens Healthcare AG
- bioMerieux SA
- Zoetis, Inc.
- Instrumentation Laboratory
- Nova Biomedical
- Quidel Corp.
- Trividia Health, Inc.
- Sekisui Diagnostics
- Nipro Corp.
- Trinity Biotech
- Orasure Technologies, Inc.
- Spectral Medical, Inc.
Order a free sample PDF of the Point Of Care Diagnostics Market Intelligence Study, published by Grand View Research.

No comments:
Post a Comment