In Vitro Diagnostics Industry Overview
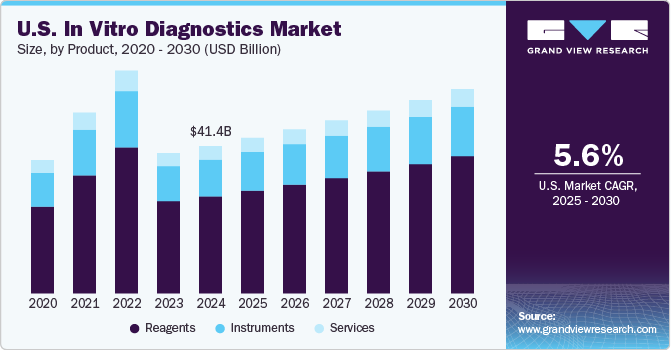
The global in vitro diagnostic market size was valued at USD 111.67 billion in 2021 and is projected to grow at a compound annual growth rate (CAGR) of 0.2% from 2022 to 2030. The growth can be attributed to the increasing adoption of IVDowing to the rise in testing due to the pandemic. The development of automated IVD systems for laboratories and hospitals to provide efficient, accurate, and error-free diagnoses is expected to fuel the market growth. The rising number of IVD (in vitro diagnostics ) products being launched by key players is also fueling the growth of the market. IVD products with molecular diagnostic capabilities deliver effective and accurate results.
In May 2021, an ultrasensitive molecular test was developed by the University of California. This test is based on a chip technology that can detect the presence of influenza A and SARS-CoV-2 antigens. The test is under further study for conversion into a Point-of-Care (POC) test. There has been a shift in the industry dynamics, with the increasing number of players focusing on launching tests for home-based testing. Moreover, in 2021, the FDA also prioritized home-based molecular diagnostic tests. In March 2021, BATM Advanced Communications Ltd. announced the launch of its molecular diagnostics self-test kit for the detection of COVID-19.
Gather more insights about the market drivers, restraints, and growth of the Global In Vitro Diagnostics market
Moreover, these tests enable the early detection of diseases, maintaining a low threat of substitutes. However, the high prices of these tests are expected to encourage patients to shift to external substitutes. Moreover, for the detection of newer infections, such as SARS-CoV-2, the rate of internal substitution is high, which boosts competitive rivalry. The penetration of IVD products from established players is low in regions, such as APAC; this region is highly dominated by local players selling instruments at lower costs. Technological advancements in terms of portability, accuracy, and cost-effectiveness are projected to be among the major drivers of this market.
The rise in the geriatric population and growth in knowledge regarding early testing has led to a surge in the number of regular check-ups, as a majority of deaths due to infections and chronic conditions occur in the population aged over 75 years. As per the Office for Budget Responsibility, U.K., healthcare costs have risen exponentially, which can create economic pressure on rapidly aging nations. However, this expenditure is anticipated to translate positively for the IVD industry, driving the market growth.
Browse through Grand View Research's Clinical Diagnostics Industry Research Reports.
In-vitro Toxicology Testing Market - The global in-vitro toxicology testing market size was valued at USD 28,014.0 million in 2022 and is projected to expand at a compound annual growth rate (CAGR) of 11.10% from 2023 to 2030.
Vacuum Blood Collection Tube Market - The global vacuum blood collection tube market size was valued at USD 2.98 billion in 2022 and is anticipated to expand at a compound annual growth rate (CAGR) of 3.02% from 2023 to 2030.
In Vitro Diagnostics Market Segmentation
Grand View Research has segmented the global in vitro diagnostics market based on product, technology, application, end-use, test location, and region:
IVD Product Outlook (Number of Instruments Installed in Thousands, Number of Reagents Sold in Millions; Revenue, USD Million, 2018 - 2030)
- Instruments
- Reagents
- Services
IVD Technology Outlook (Number of Instruments Installed in Thousands, Number of Reagents Sold in Millions; Revenue, USD Million, 2018 - 2030)
- Immunoassay
- Hematology
- Clinical Chemistry
- Molecular Diagnostics
- Coagulation
- Microbiology
- Others
IVD Application Outlook (Revenue, USD Million, 2018 - 2030)
- Infectious Disease
- Diabetes
- Oncology
- Cardiology
- Nephrology
- Autoimmune Disease
- Drug Testing
- Others
IVD End-use Outlook (Number of Instruments Installed in Thousands, Number of Reagents Sold in Millions; Revenue, USD Million, 2018 - 2030)
- Hospitals
- Laboratories
- Home Care
- Others
IVD Test Location Outlook (Revenue, USD Million, 2018 - 2030)
- Point of Care
- Home Care
- Others
IVD Regional Outlook (Number of Instruments Installed in Thousands, Number of Reagents Sold in Millions; Revenue, USD Million, 2018 - 2030)
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)
Market Share Insights
January 2021: Abbott received FDA approval for its rapid handheld Traumatic Brain Injury (TBI) blood test. The first-of-its-kind test is used for assessing mild TBIs and concussions in patients.
March 2020: Abbott launched the RealTime SARS-CoV-2 assay, a PCR-based test, for the diagnosis of COVID-19, and BioMedomics launched a point-of-care COVID-19 test that can detect antibodies in blood within 15 minutes.
Key Companies profiled:
Some of the prominent players in the In Vitro Diagnostics market include:
- Abbott
- Bio-Rad Laboratories, Inc.
- Quest Diagnostics
- bioMérieux SA
- QIAGEN
- Sysmex Corp.
- Agilent Technologies
Order a free sample PDF of the In Vitro Diagnostics Market Intelligence Study, published by Grand View Research.

No comments:
Post a Comment